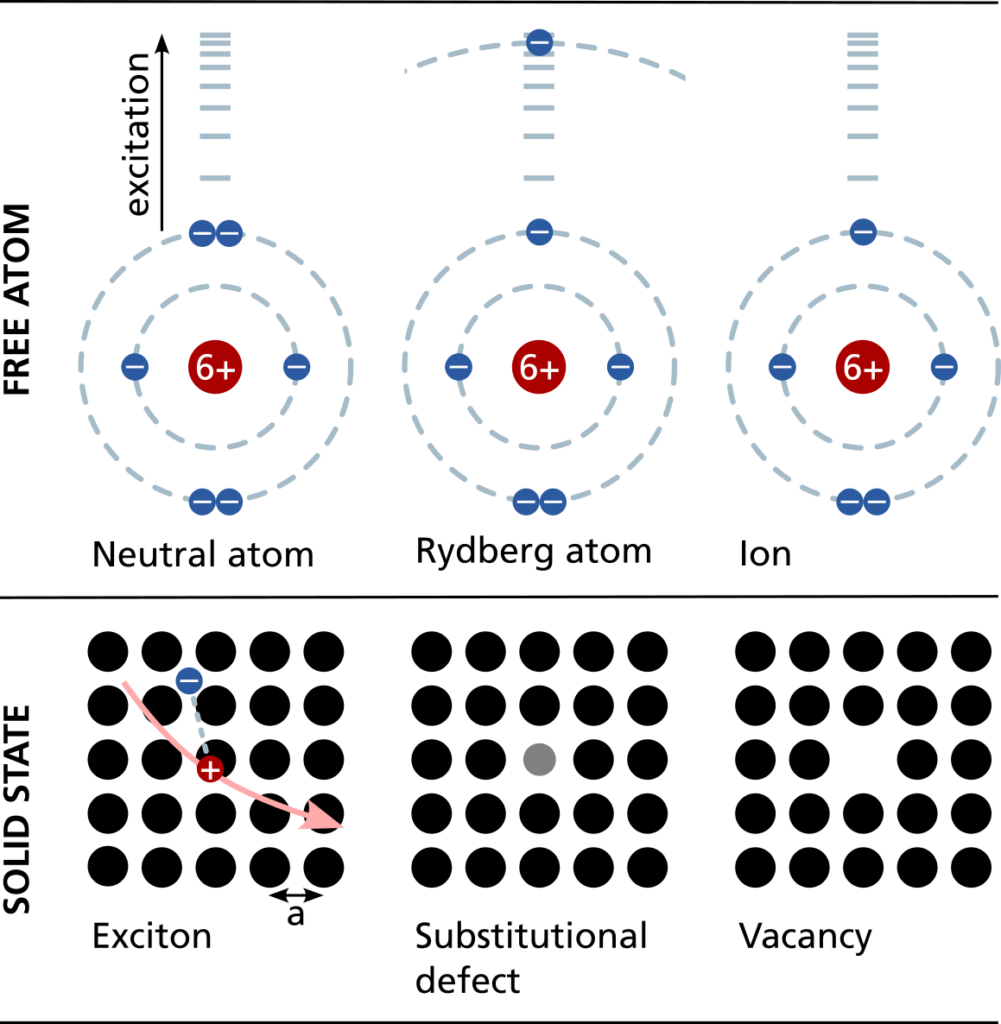
This page deals with the properties of excitable single free atoms and of point defects inside solid state matter. The properties of molecules as information carriers are discussed separately. Single-atom transistors use electrons as information carrier and thus fall into the category of unconventional electronics.
Naturally, atoms and point defects have very small dimensions (radius <0.5 nm). Excitons can reach several nanometers of radius. All of them have several energetic levels to which an electron can be excited to by radiation. Some of them become distinguishable in presence of a magnetic field due to the Zeeman splitting which separates them in their energy. When the electron is excited to a high energy level, the atom is called a Rydberg atom. Then, the electron has a large mean distance to the nucleus of the atom which causes a dipole moment. If the excitation energy is increased beyond its binding energy, the electron is separated and the atom becomes an ion. Further, both electrons and nucleus of an atom obtain a degree of freedom called spin.
Free Atoms
Neutral Atoms
A group of or even individual atoms can be trapped by optical tweezers. A specific electron can be excited by using laser pulses matching the excitation energy. If the electron is excitated to a high energetic state it is called a –> Rydberg atom .
Features
- neutral particle
- weak interaction with each other
- volatile without power for trapping
- ultimately small dimension
- characteristics are identical for each individual atom –> reproducible results
Rydberg Atoms
Rydberg atoms are –> neutral atoms, where the electron is excited to a high energetic state by light or incoming electrons, which changes some features significantly. As the other electrons shield the nuclear charge from this highly excited electron, it is subject to an effective potential similar to the one of hydrogen.
Features
- neutral particle
- large dipole moment –> highly sensitive to electric and magnetic fields
- strong van der Waals interaction between near Rydberg atoms
- very small dimension
- characteristics are identical for each individual atom –> reproducible results
- used as qubits with relatively slow quantum gate operation
Ions
Ions are charged atoms and can therefore be trapped by electrical and/or magnetic fields besides of optical tweezers. This charge also leads to strong Coulomb interactions between them, but also allow a simple manipulation by electric and magnetic fields. Thus, besides their individual usage, they can flow in transistors and memories to transport charge or build conductive paths.
Features
- charged particle –> can be trapped by electrical and/or magnetic fields and be used to transport current
- strong interactions between ions
- ultimately small dimension
- characteristics are identical for each individual atom –> reproducible results
- used as qubits with relatively slow quantum gate operation
Common variables of free atoms for encoding information
- position in space
- excitation state
- spin of valence electron –> page magnetism/spin
- nuclear spin –> see page magnetism/spin
Solid State
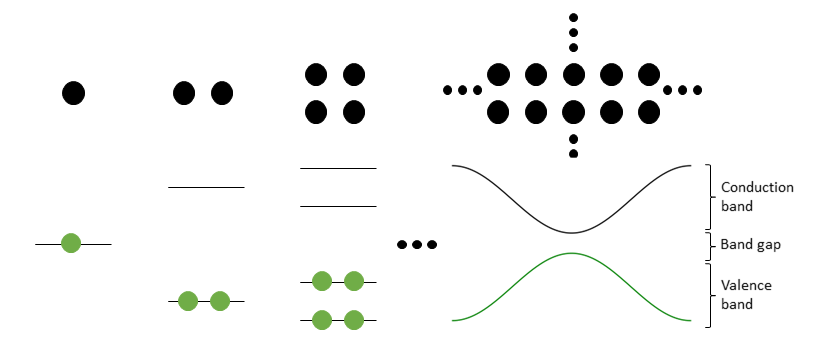
Solids consist of are a large number of atoms which are arranged in a regular lattice. The atoms arrange in such a way, that their electronic structure has the lowest energy. This in turn is a result from the hybridization of electronic orbitals accompanied by a mixture of sharing and transfering electrons. The hybridization of this large number of atoms then leads to a continuous range of electronic states called “bands”, schematically displayed in figure 2. Bands, which are filled with electrons, are called valence bands, while empty bands are called conduction bands. A material is a metal if there is a band which is partially filled such that no energy gap between occupied and free states exists, while a band gap appears in semiconductors and insulators.
Excitons
An exciton is a specific kind of electron-hole-pair, created in an insulator or semiconductor when a photon is absorbed. The electron is excited from the valence band to the conduction band, leaving behind a positively charged “hole” in the valence band. The electron and the hole remain bound to each other by the Coulomb interaction, forming a quasi-particle with dipole moment called “exciton”.
Features:
- neutral quasi particle
- large dipole moment
- small dimension
- Spin-0 or Spin-1
- can be mobile or trapped at an atom
- short lieftime
Origin of positive and negative charges: Mott hubbard and spin-orbit types
Variables of excitons for carrying information
- Spin
- Excitation energy
- Position
- Momentum
Spin: Excitons can have a definite spin, which can be used to encode binary information. For example, in some materials, the spin of an exciton can be used to represent the state of a qubit.
Energy: Excitons can have different energies, which can be used to encode information. For example, by controlling the energy of excitons, it is possible to create a set of states that can be used to represent the state of a qubit.
Position: Excitons can be localized at specific positions within a material, and the position of an exciton can be used to encode information. For example, by controlling the position of an exciton, it is possible to create a set of positions that can be used to represent the state of a qubit.
Momentum: Excitons can have a definite momentum, which can be used to encode information. For example, by controlling the momentum of excitons, it is possible to create a set of states that can be used to represent the state of a qubit.
Point defects
Substitutional Defects
Substitutional defects occur when the usual atom at a lattice site is replaced by another chemical element, i.e. an impurity. If the atom has more valance electrons than the other atoms, it is a “donator” and donates the excess electron to the host crystal thus becoming positively charged. If the atom has less valance electrons than the other atoms, it is an “acceptor” and recieves an additional electron from the neighboring atoms and thus becomes negatively charged. Donor/acceptor atoms are important for doping of semiconductors. Single donor/acceptor atoms are also utilized to store information in their nuclear spin (–> see page Magnetism / Spin).
Interstitial Defects
Impurity atoms can also be incorporated to interlattice positions of a crystal. These defects are rarely applied for computing purposes (due to their limited degrees of freedom to decode information and a high susceptibility for moving and environmental noise.)
Vacancy
A vacancy is a lattice site in a crystal lattice, that is not occupied by an atom. When a pair of oppositely charged atoms is missing in an ionic crystal this is called Schottky defect. Atoms that move from their original spot (creating a vacancy) to an interstitial site create a Frenkel defect.
Color Centers
Color centers are point defects or a cluster of defects in a crystal lattice, which results in an unpaired electron or hole trapped in the defect. This electron/hole can be excited by the absorption of photons, often in the visible spectrum, causing color in an otherwise transparent material, leading to the name color center. One such defect of technological relevance for quantum sensing and quantum computing is the NV- center in diamond, where a substitutional nitrogen sits next to a carbon vacancy.
Features:
- Localized
- Very Stable
- Small dimension
Common variables of point defects for carrying information
- Energy levels of excited electron/hole
- Spin state
- Charge state
Literature
| Title | Author | Year |
| Natural and artificial atoms for quantum computation | Iulia Buluta, Sahel Ashhab, Franco Nori | 2011 |
| Quantum computing with atomic qubits and Rydberg interactions: progress and challenges | M. Saffman | 2016 |
| Trapped-ion quantum computing: Progress and challenges | Colin D. Bruzewicz et al. | 2019 |
| Material platforms for defect qubits and single-photon emitters | Gang Zhang et al. | 2020 |
| Semiconductor qubits in practice | Anasua Chatterjee et. al. | 2021 |